
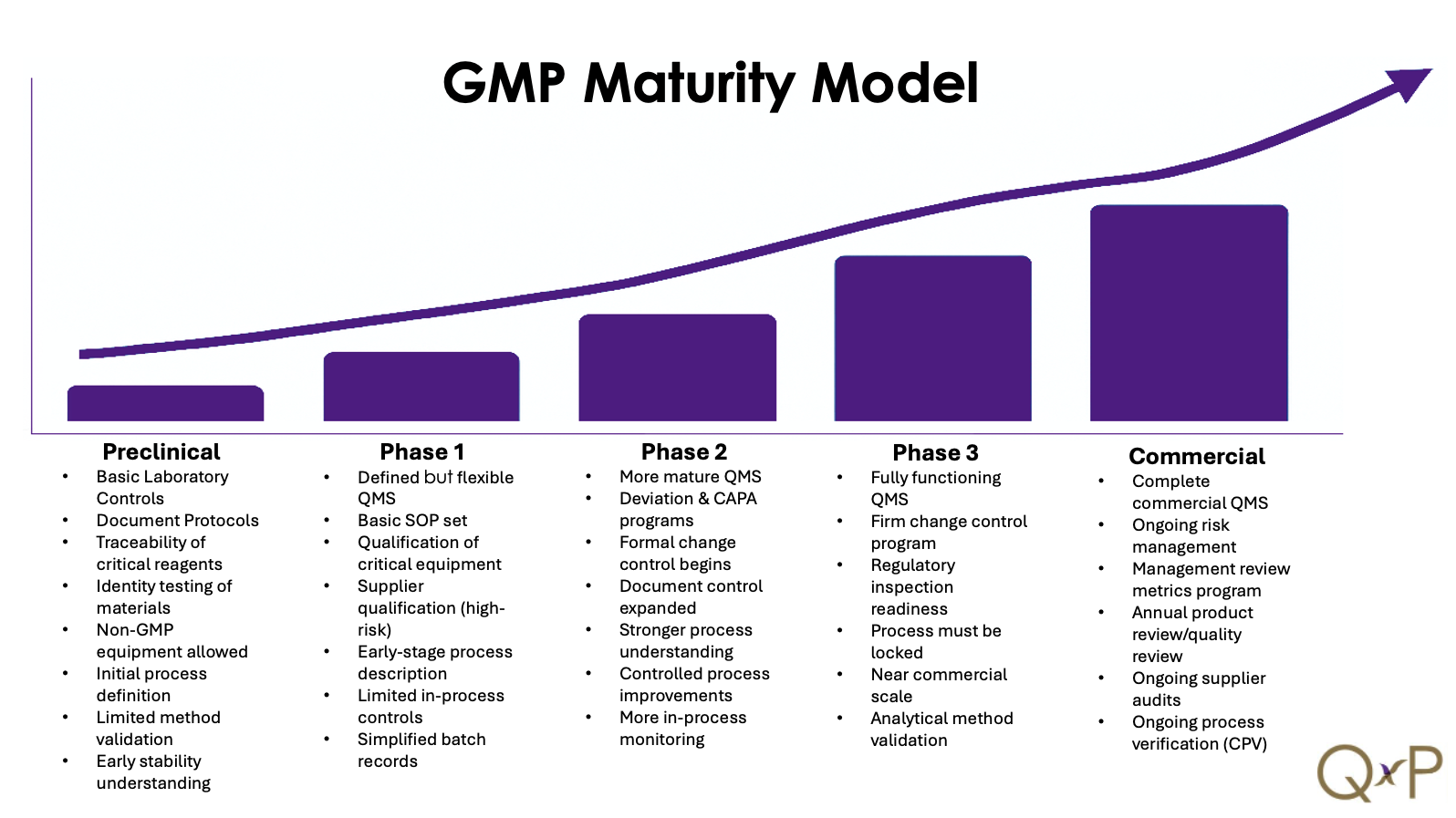
Implementing a phase-appropriate GMP (Good Manufacturing Practice) system from clinical development to commercial production presents a range of technical, regulatory, and operational challenges, especially in highly regulated industries like pharmaceuticals and biotech. Below are the key challenges:
1. Scalability and Process Evolution
- Early clinical phases (Phase I/II) focus on small-scale, flexible processes to support rapid iteration and safety assessments.
- As development progresses, processes must scale for Phase III and commercial supply, often requiring technology transfer, process validation, and equipment upgrades.
- Challenge: Balancing process flexibility in early phases with the robustness and reproducibility required for commercial manufacturing.
2. Evolving Regulatory Expectations
- GMP requirements increase with each phase:
- Phase I: Emphasis on documentation and patient safety.
- Phase III: More stringent controls, qualification of equipment, and data integrity.
- Commercial: Full compliance with ICH Q8–Q10, FDA/EU GMPs, and inspection readiness.
- Challenge: Implementing systems that are compliant yet scalable, without over-investing too early.
3. Quality System Maturity
- Early-stage companies often operate with lean quality systems.
- Transition to later phases requires:
- Formal Change Control
- Deviation and CAPA management
- Supplier qualification
- Robust document control and training
- Challenge: Scaling the quality management system (QMS) without disrupting operations or delaying timelines.
4. Data Integrity and Electronic Systems
- Manual or hybrid systems may suffice in early stages but become inadequate for handling the volume and complexity of data in late-stage development.
- Transitioning to validated electronic systems (e.g., LIMS, QMS, MES) is resource-intensive.
- Challenge: Timing and validation of these systems while ensuring continuity of compliance.
5. Technology Transfer and Site Readiness
- Moving from clinical to commercial often involves:
- Transferring processes to CMOs/CDMOs or in-house commercial sites
- Ensuring consistent documentation, training, and equipment qualification
- Challenge: Maintaining GMP continuity and comparability of product through each transfer.
6. Cross-Functional Coordination
- Requires alignment across R&D, Quality, Regulatory, Manufacturing, and Supply Chain.
- Challenge: Avoiding silos and ensuring that decisions are made with an understanding of future commercial implications.
7. Cost and Resource Allocation
- Phase-appropriate GMP aims to optimize resources by applying only what’s necessary for a given phase.
- However, under-investing early may lead to costly rework or regulatory delays later.
- Challenge: Finding the right balance between risk and compliance.
8. Cultural and Organizational Readiness
- Start-ups or early-phase companies often have a research-centric culture.
- Moving to commercial requires a compliance-driven culture, formal training, and operational discipline.
- Challenge: Managing change management and training across teams and sites.
Achieving full GMP compliance through validated, reproducible processes and systems—while ensuring alignment with regulatory expectations for commercial readiness is the most challenging journey you will take. QxP has many experts to help take you there. We have helped many to achieve their commercial success quicker and more cost effective than doing this alone. Call us!

